The History of Iodine in Japan
1.Introduction
The history of iodine in Japan began about 30 years after its discovery in France in 1811. Japan has known iodine for a long time, with Japanese scientists or manufacturers engaged in collecting materials, extracting half-finished products, producing final products (disinfectant/antiseptic, medicines, industrial raw materials, and so on). Although Japan lacks underground resources, it boasts the world’s largest reserves of iodine. I hope further work on iodine will contribute to the development of science and industry. I believe this article, which examines the history of iodine in Japan, will be helpful to the people involved.
2.The dawn of iodine in Japan
2-1 Edo period
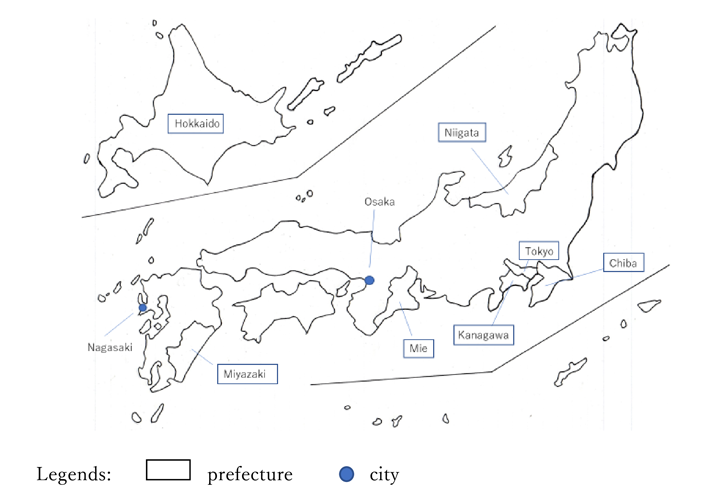
Iodine came to Japan by import into Nagasaki in 1842(1). Although Japan was still a closed country (because of its national isolation policy), the discovery of iodine in France in 1811 led to its spread to Japan in about 30 years.
By this time, iodine was a common medicine in Europe. It is believed that it was transmitted to Nagasaki, which was Japan’s only window to Western Europe.
Then, in 1846, a doctor in Edo (Tokyo), Ryuho Shima , succeeded in extracting iodine. We find this in external-book volume 1 of Seimi-Kaiso(2), which is said to be Japan’s first chemistry book. I believe he is the pioneer of domestic iodine in Japan.
Under these circumstances, from the end of the 1840s to the mid-1850s, some people brought seaweed ash, an intermediate material, to Yokohama from Mie . ”The Report of Important Products in Each Prefecture”(3) states, “People learned to make medicine from seaweed. They burned eisenia or ecklonia kelp and brought them to Yokohama.”
Interestingly, around 1857, Yukichi Fukuzawa was trying to extract iodine at Tekijuku, which was a private school headed by Koan Ogata. Examining Fukuzawa’s autobiography (4), “We investigated various books to make iodine, went to greengrocer’s market in Temma, bought kelp, and roasted it. But we could not do it, though our hands became black with ash at all.” It seems it was a failure after all; however, this episode gives a glimpse of the strong curiosity about foreign medicine, pharmacy, and chemistry at that time.
Here, I would like to explain a general method for producing iodine at that time. Brown alga, such as eisenia or ecklonia are dried and smothered to seaweed ash. They are leached with hot water, concentrated, and cooled. Then, sulfuric acid and manganese dioxide are added to liberate iodine.
The discovery of iodine in France was a coincidence. Too much sulfuric acid was added when taking out potassium chloride (raw material for gunpowder). Subsequently, iodine crystals were obtained (5).
Efforts to produce iodine in Japan continued to progress steadily, and in 1861, Genboku Ito, a doctor in the shogunate, produced iron iodide, and other products, at the Ninomaru Manufactory in the shogunate in Edo. At that time, various medicines were coming into Japan from the Netherlands, and there was momentum to make them domestically. Ito repeatedly submitted manufacturing requests to the shogunate and was eventually granted permission (6). Ito was also involved in the examination of Shogun Iemochi Tokugawa and his wife Kazunomiya.
2-2 After the Meiji Restoration to the beginning of the 20th century.
Around the Meiji Restoration, some people gradually began producing iodine as a business. “The history of medicine-50years” (7) states that “A man named Seisuke Rokkado built a factory in Edo-Benkeibashi to manufacture iodine and mercury chloride solution.” In addition, it is said that a Frenchman who stayed on the coast of Tateyama in Chiba taught the local fishermen to produce iodine at the start of the Meiji era (8). We find iodine production using seaweed start in various places during this time.
In Hokkaido, the first activity began around Kushiro. “The survey on iodine and potassium chloride production in Hokkaido” (9) states, “Mr. Manpei Kashima dispatched engineers around mid-1870 for the trial manufacturing of iodine at Hamanaka in Atsukeshi county, Kushiro. It was the first trial in Hokkaido.” Furthermore, around 1879, Manpei Kashima started production in Atsukeshi, but stopped as he was unable to make profits.
This survey document also states, “In 1880, The Hokkaido Development Commission collected about 17 kilo liters of kelp and made about 27kg of iodine at Shukutsu, Takashima county, Shiribeshi. However, it was just a one-time trial.” According to the book, efforts to produce iodine continued in Hokkaido, where seaweeds were abundant. Around 1887, seaweed ash was made in the Kushiro region, and transported to Hakodate and Tokyo, where the mother liquor (from seaweed ash) was produced and sold. Furthermore, several manufacturing sites were established in Hokkaido around 1891.
Hokkaido was the center of efforts in iodine production because it had abundant seaweed and the encouragement of the Hokkaido Development Commission, which was the national institution. This commission was established in 1869, and one of its major themes was the export promotion of essential goods such as salted products, canned foods, smoked products, cod-liver oil, and iodine. In line with such basic policy, manufacturing and trial production were done in various places in Hokkaido.
We find the the germination of iodine production in Mie prefecture, too. According to “Fishery Journal, Vol.7” (10), Seikichi Okamoto, Sakubei Yamamoto in Shima county tried to produce iodine using a recipe of iodine production, obtained from a person named Taizo Tamagawa in 1884. Although an iodine factory was built in 1888, it was difficult to raise funds because of the lack of understanding of the surroundings.
Efforts to produce iodine were ongoing in several places in Japan. From the business perspective, Chujiro Kase was the first successful iodine producer in Japan. He constructed the Kase Iodine Factory at Irifune-cho Fukagawa in Tokyo in 1887.
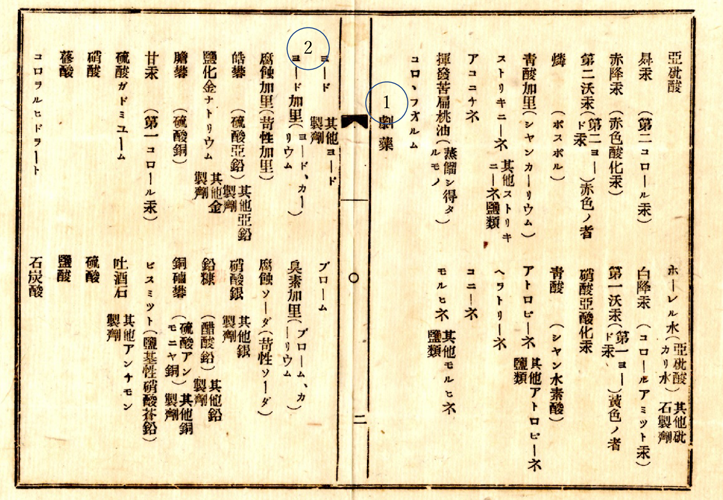
When the domestic production of iodine began, the government enacted the Poisonous and Deleterious Substances Control Act under Tomomi Iwakura, the deputy prime minister, in 1877, in response to the arrival of various chemicals, including iodine, in Japan(Photo 1). This is a historical act, which continues to this day. Subsequently, Japanese Pharmacopoeia was promulgated. This document listed, iodine and iodine compounds, iodine preparations, and so on, from the beginning (11). Thus, a system for handling iodine was established early on.
Photo 1 : The Poisonous and Deleterious Substances Control Act
The words “Deleterious Substances” is in the center of the spread(①). “Iodine and potassium iodide” are on the left of it(②).
(Notice issued by the Governor of Yamagata Prefecture in February 1877 under the name of Deputy Prime Minister Tomomi Iwakura. The specific notice seems to have been given by each Governor. )
[Collection of The Society of Iodine Science]
Iodine tincture (disinfectant/antiseptic made by dissolving iodine in alcohol) was a typical application in this era. The production of iodine tincture started in the latter half of the 1870s in Japan.
Under these circumstances, the iodine business continued to expand. In 1890, in Doshomachi Osaka, Chobei Takeda, Gohei Tanabe, and Gisaburo Shiono (CEOs of [today] Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Shionogi & Co., Ltd, respectively) established Kogyosha and started iodine production. Subsequently, Kogyosha was renamed the Kogyo Joint Stock Company, and it purchased seaweed and seaweed ash in the Shima area of Mie prefecture and used it to make iodine (12).
In 1892, a production manual called “Fisheries, Craft, Iodine Production Book” (13) was issued. It seems to have been a catalyst for the flourishing of iodine production in various regions.
In 1893, Toragoro Tanahashi in Tokyo acquired a patent for the potassium iodide manufacturing method, and established the Azabu Yosho Joint Stock Company. In Kanagawa, the Suzuki family, which was recommended by Shunrei Murata, an engineer at Dai-Nippon Pharmaceutical Joint Stock Company, started producing iodine in 1888. In 1893, the Suzuki established Suzuki Seiyakusho (today, Ajinomoto Co.,Inc.) in Hayama. In the same year, Kahei Tomoda, who was a large drug wholesaler, established the Osaka Jitsugyo Joint Stock Company and started working in the iodine business.
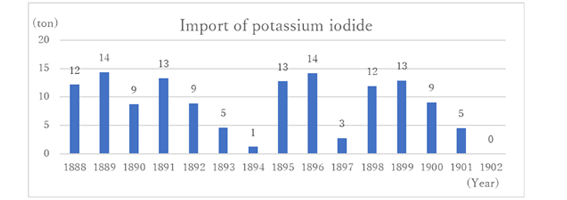
Thus, by the Sino-Japanese War of 1894, Japan’s domestic production was sufficient, and it became almost unnecessary to import iodine.
However, iodine companies from the UK, Germany, and Chile joined together in an attempt to crush the emerging Japanese iodine business, and began a violent low price offensive in 1896. Trading companies in Japan, which connected business, were divided to a Japanese side group and a foreign side group. The price of iodine temporarily dropped to half. Overseas corporations tried to acquire the iodine business across Japan. To do this, foreign companies had to persuade small businesses scattered from Hokkaido to Kyushu one by one. However, Japanese companies suddenly stopped production when iodine became cheap, and resumed production when prices increased, and sold it cheaper than imported goods. Thus, the acquisition attempts of foreign companies failed (7). After 1902, Japan did not import iodine (Graph 1).
Graph 1:From “Chemical industry Compendium Book 1”(14) data
The import statistics at that time recorded data on potassium iodide, not iodine itself. It is estimated that iodine was mainly imported in the form of potassium iodide, which is soluble in water and easy to handle, for use as medicines and as disinfectants/antiseptics.
However, in such fierce competition, when iodine prices fluctuated, the Azabu Yosho Joint Stock Company ceased manufacturing iodine products, and in 1904, it was transferred to the Suzuki family’s Saburosuke Suzuki.
In 1901, Nobuteru Mori, who would later play an important role in the iodine business, began an apprenticeship at the Ikehira Crude Iodine Factory. In 1903, significant iodine from all over Japan was exhibited in the 5th Expo in Osaka. These movement predicted the future development of the iodine business.
3.Development of the iodine industry
3-1 Boom due to wars
The Russo-Japanese War and World War I expanded demand for iodine, as disinfectants/antiseptics, and demand for potassium chloride. Potassium chloride was a co-product of iodine, used as raw material for explosives (potassium nitrate, which was the primary material in explosives at that time, was made by reacting potassium chloride with sodium nitrate). These wars brought about the development of the iodine business.
3-2 Industry reorganization after the Russo-Japanese War
In 1906, Saburosuke Suzuki of the Suzuki family established the Awa Iodine Factory in Tateyama in Chiba prefecture, and established a manufacturing company in Mie prefecture (15). Saburosuke’s passion for iodine can be seen from his involvement in major iodine producing regions, such as Kanagawa, Chiba, and Mie.
Meanwhile, also in 1906, the Kanto Iodine Association was formed to deal with the temporary iodine recession after the Russo-Japanese War. However, it was dissolved without any significant results. The Mie Iodine Association, which was created for the same purpose, later developed into Mie Iodine Manufacturing Co., Ltd. During this period, Chujiro Kase, Toragoro Tanahashi, and Saburosuke Suzuki jointly established Nippon Chemical Industrial Co., Ltd to produce iodine and its related products [in 1907]. Three major producers in the nascent stages of the iodine industry in Japan combined(however, only the Azabu factory joined from the Suzuki family and not the Hayama factory. The latter developed independently as Suzuki Seiyakusho Co.).
In 1908, Nobuteru Mori established Sobo Marine Products K.K. This company competed violently with Saburosuke Suzuki’s Awa Iodine Factory. However, Saburosuke deliberately avoided the decisive battle and merged Awa Iodine Factory into Sobo Marine Products. Thus, Sobo Marine Products became the dominant company in Chiba prefecture.
Thereafter, Mie Iodine Association was dissolved, and Mie Iodine Manufacturing Co., Ltd. was established headed by Enkichi Ishihara in 1912. Consequently, the business development of iodine was primarily undertaken by one leading company in Mie prefecture.
3-3 Evolution of the monopoly system for by-products
There was one characteristic change in the Japanese iodine industry after the Russo-Japanese War, that is, the start of the monopoly system for salt, which is a by-product of iodine. At that time, the mainstream process for obtaining crystalized iodine was concentrating and cooling the extract of seaweed ash in a distillation still. Finally, the precipitated sodium chloride was removed. The monopoly system was established to raise war costs for the Russo-Japanese War; however, it also had a considerable impact on the iodine industry. Specifically, the Monopoly Bureau of the Ministry of Finance urged the iodine industry to join forces for the stable management of salt manufactures and ease of administration by the authorities.
The establishment of Nippon Chemical Industrial Co.,Ltd. was said to have been mediated by the Monopoly Bureau. When Sobo Marine Products was established, Nobuteru Mori provided his own plants free of charge to persuade peers based on the idea of this bureau (16).
Collecting and drying seaweeds (such as ecklonia or eisenia) was a valuable source of income for fishermen (Photo 2). However, it impeded the stability of raw material procurement, because the amount of seaweed collected was influenced by the balance with other fishing work that fishermen had to do.
Photo 2:Collecting and drying seaweeds
From [Illustrated Chemical Industry](17)
Photo on the right shows seaweed collection.
Photo on the left shows drying seaweed on the beach.
3-4 Impact of World War I
Under these circumstances, Nippon Chemical Industrial Co., Ltd, Suzuki Seiyakusho Co., Sobo Marine Products, and Mie Iodine Manufacturing Co., Ltd., among others, were the iodine manufacturers in Japan. However the iodine business in Hokkaido, which was initially active, was not faring well.
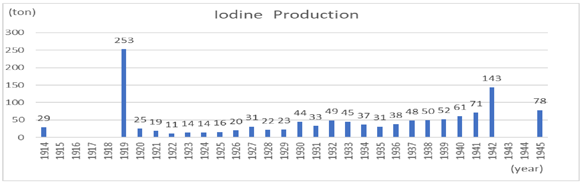
While the industry was consolidating into influential companies, the Japanese iodine business was booming sometime after the beginning of World War I. As Japanese iodine was an export industry, the decrease in supply from Europe acted as tail wind.
Japan’s annual iodine production in 1914 was 29 tons per year. It reached 253 tons in 1919, after the end of World War I.
However, the reactionary recession after the war was severe. The offensive with low prices from overseas was tremendous. In 1922, Japanese production dropped to 11 tons.
Under such circumstances, Nobuteru Mori’s Sobo Marine Products faced a great business crisis. It was merged with Toshin Denki Co.,Ltd, an electrochemical company owned by Saburosuke Suzuki, in 1919. In Toshin Denki, Nobuteru Mori became a director of fishery, and later, a director of power plants construction. Thereafter, he got iodine factories in Chiba prefecture and established Nihon Iodine K.K. (today, Showa Denko K.K.) in 1926. He focused on the iodine business again. He signed a mass contract with the Soviet Union, established the Sakhalin Iodine Joint Stock Company, and Korea Iodine K.K. Thus, he expanded iodine business. Initially, semi-annual business results also grew steadily; however, they did not last long. The recession following the Showa financial crisis of 1927 brought a significant decrease in profits and forced capital reductions.
3-5 Industry hardship and increasing production for World War II
Under these circumstances, Nobuteru Mori shifted to the electro -chemical industry, perticularly fertilizers. His business changed to that of Showa Denko.
In addition, Saburosuke Suzuki gradually focused on the “Ajinomoto” business, rather than the iodine-based business.
Saburosuke Suzuki left Nippon Chemical Industrial Co., Ltd. a few years after its establishment. Chujiro Kase passed away at the end of the Meiji era. Therefore, Toragoro Tanahashi became the central figure in the management, but his interests moved to inorganic chemicals other than iodine.
Meanwhile, in Mie prefecture, the Ise Iodine Factory (later, Ise Chemicals Corporation) was founded in 1927 by the Ise capitalist who ran a shipping company. Nihon Iodine K.K. also established a factory in Ise Shima.
However, the recession was unstoppable. The Great Depression in 1929 impacted Japan’s iodine business further. In Mie prefecture, iodine production began to decrease rapidly around 1932. All the factories stopped production in 1935, including Mie Iodine Manufacturing and Ise Iodine Factory. However, the history of the pharmacy business in Mie prefecture states that “There was a conflict with the management of salt under the monopoly law. The restrictions were imposed by the authorities, and the business was affected.” (18) It is speculated that there was an institutional obstacle.
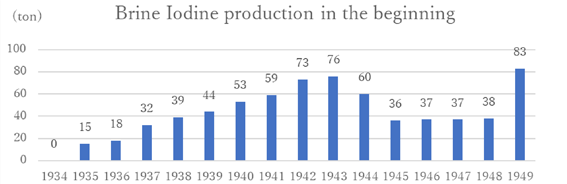
Thus, the production that seemed to recover from the recession after World War I, dropped to merely 31 tons in 1935. It gradually increased again before World War II. Ultimately, when the world was at war again, the production of iodine reached to the peak.
The period from the end of the Meiji era to the end of World War II showed large differences between peaks and bottoms. The Iodine industry in Japan had developed significantly during World War I. However, the post-war recession and the low-price offensive from overseas drastically reduced production. Iodine production did recover slightly, but the Showa financial crisis and the Great Depression forced a sluggish pace of business. Then, with the outbreak of World War II, production gradually increased(Graph 2).
Graph 2: From “Factory Statistics Table”(19) data
(There is lack of data because statistical surveys were not conducted in these years)
4.Change in material (Seaweed ⇒ brine water)
4-1 History of non-seaweed iodine production
Iodine from seaweed was the only method in use in Japan since the discovery of iodine. However, in 1934, iodine production from natural gas brine (ancient underground seawater with natural gas) began in Chiba prefecture.
The history of iodine production from non-seaweed is rather long. In 1862, it was discovered that oil field brine (ancient underground seawater with oil) in East Java Indonesia contained a lot of iodine. Later, concentrated and desalted mother liquor was shipped to Europe. From 1911, iodine was produced locally in the form of copper iodide. In 1868, Chile started exporting iodine as a by-product of saltpeter (20). In 1924, the Soviet Union started producing iodine from brine (21). In addition, in 1927, the extraction of iodine from spa components started in Italy. In 1928, an iodine production plant from brine was constructed in the United States (22).
4-2 Development in Chiba
While iodine production from non-seaweed continued to progress globally, Mikasa Shokai (later, Aioi Kogyo, and today, Godo Shigen Co., Ltd.) constructed an iodine factory using natural gas brine at Kamitaki in Isumi county, Chiba prefecture, in 1934(23). In 1938, Tennen Gas Kagaku Kogyo (today, Kanto Natural Gas Development Co., Ltd.) also built an iodine factory in Mobara, Chiba prefecture (24). In 1939, Nippoh Kagaku Kogyo (today, Nippoh Chemicals Co., Ltd.) constructed an iodine factory in Isumi county, Chiba prefecture(25). In 1940, Nihon Kansui Kogyo Kenkyusho, a company affiliated with Nihon Tennen Gas Kogyo (today, Nihon Tennen Gas Co., Ltd.) started iodine production in Seki Mura, Chiba prefecture(26).
Unlike production with seaweed, iodine production from brine has a stable supply of raw material (it does not depend on the procured amount of seaweed) and demonstrates good productivity in the manufacturing process. Before and in World War II, many companies began producing natural gas and iodine in the Boso area, which is rich in natural gas brine.
Ise Iodine Factory (today, Ise Chemicals Corporation), which was based in Mie prefecture, built the second seaweed iodine plant in Mie immediately after the war. However, this plant did not operate for long. In 1949, Ise Chemicals Corporation decided to move its production base to Chiba prefecture.
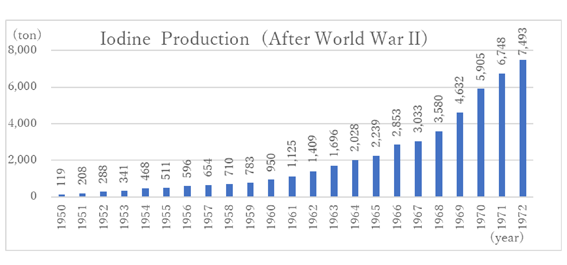
Thus, the production of brine iodine continued to increase. Although it decreased during the World War II and the post-war turmoil, it completely recovered in 1949. This led to smooth development thereafter (Graph 3). The production of seaweed iodine ended around 1950.
Graph 3: From “U.S. Geological Survey” data
4-3 Development of natural gas industry and innovation in production methods
In the era of brine iodine, various production methods such as the copper method, starch adsorption method, active carbon adsorption method, and solvent extraction method, were developed.
In particular, the copper method and starch adsorption method were developed jointly by companies, universities, and the Institute of Physical and Chemical Research (today, RIKEN). This was the precursor of collaboration between academia and business. It was necessary to gain knowledge to extract iodine at a reasonable cost, as iodine is dissolved only 100 parts per million in brine.
The post-war period until the 1960s was when the chemical industry became increasingly active in using natural gas as material and fuel. The high demand of natural gas created an environment for the mass production of iodine, as iodine was a co-product of natural gas. Under such circumstances, it was inevitable that the production volume of each plant shifted from several tons per month to several tens of tons per month. In such situations, the blow-out method, which was developed by an affiliate company of Dow Chemical Corporation in the United States in 1928, was highlighted. This method is, in principle, suitable for mass production, as it uses a sublimation property of iodine. When oxidizer is added to brine, it releases iodine from brine. Then, this vaporized iodine is reduced and concentrated. The process of “Sublimation of iodine by contacting brine with air” seems to be the reasoning for the expression ” blow-out”. However, for some reason, the theoretical efficiency could not be achieved in the actual plant.
However, this method of extracting bromine from seawater was adopted quickly in business. In 1934, it was put into practical use by Dow Chemical Corporation. In Japan, Toyo Soda Manufacturing Co., Ltd (today, Tosoh Corporation) first adopted it for large-scale production for a military order in 1941. In addition, the Makiyama Factory of Mitsubishi Kasei Co., Ltd. (today, AGC, Inc.), and the Omuta Factory of Mitsui Chemical Industries Co.,Ltd.(today, Mitsui Chemicals, Inc.), and so on, began using this production method. However, as the primary use of bromine at that time was as an anti-knocking agent for military aircraft, factories were forced to stop at the end of World War II (27).
The blow-out method to produce iodine in Japan was attempted in 1952 by Otaki Gas Co., Ltd.(today, Kanto Natural Gas Development ), and by Nihon Tennen Gas Kogyo(today, Nihon Tennen Gas). However, according to the history books of both companies, those plants were small-scale or remained experimental.
Under these circumstances, Asahi Glass Co., Ltd.(today, AGC) undertook capital participation in Ise Chemicals Corporation in 1960. Asahi Glass and Ise Chemicals collaborated on the development of the blow-out method, as Asahi Glass had the knowledge to extract bromine from seawater using the blow-out method. An individual who understood the blow-out process for bromine became the chief of this development. After careful study of the mechanism through chemical engineering, Ise Chemicals adopted the blow-out method full-scale in 1961(Photo 3).
Photo 3 : A pilot Plant installed at Shirasato Plant, Taiyo Kagaku Co.,Ltd.
(today, Shirasato Plant, Ise Chemicals Corporation)
It was a small facility with a
height of about 9 m.
[Provided by AGC Inc.]
In 1964, Nippoh Kohatsu Co., Ltd (today, Nippoh Chemicals) also installed a test plant and put it use. In 1973, Godo Shigen adopted the blow-out method, too. Furthermore, the blow-out method was positioned as the major iodine production method in the industry, as it was successively deployed in overseas companies.
Around the same time, several companies began developing iodine extraction using ion exchange resin. Subsequently, it was also adopted at the actual plant level.
4-4 Increasing production to the top of the world
Under such circumstances, the number of iodine businesses was increasing. In 1966, Nihon Halogen Co., Ltd.(today, JX Nippon Oil & Gas Exploration Corporation) took brine from the Nihon Kogyo Nakajo Oil Plant in Niigata prefecture, and started iodine production. Nihon Halogen was invested in and established by Nippoh Kohatsu and Nikko Consultant Co.,Ltd. In 1970, Teikoku Oil Co.,Ltd (today, INPEX Corporation) entered the iodine business. In 1995, Toho Earthtech,Inc. started production of iodine in Niigata prefecture(28). It was common knowledge that Miyazaki prefecture possessed natural gas brine deposits. Ise Chemicals Corporation started iodine production in the Sadowara area of Miyazaki prefecture in 1975.
Therefore, by the 1990s, the iodine supply system was set up by eight companies in three regions: Chiba, Niigata, and Miyazaki.
Consequently, Japan’s iodine business was supported by the development of the natural gas industry, technological innovation in iodine production, increase in iodine producers, and the expansion of iodine to various uses. It showed remarkable growth within 20 to 30 years after the war (Graph 4).
Graph 4: From “Chemical Industry Statistics,
Ministry of International Trade and Industry” data
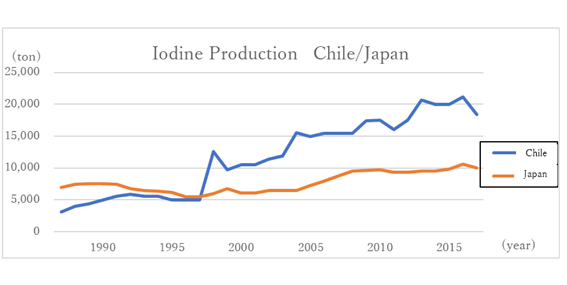
Globally, the production of iodine from Chilean saltpeter, which started in the middle of the 19th century, had grown to account for the majority of the world market in the 20th century. However, the Japanese production of iodine exceeded that of Chile in 1967, making Japan the No.1 iodine producing country in the world. It held onto this position for about 30 years, until 1997.
4-5 Technology development in Japan
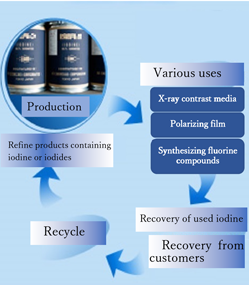
The practical application of the blow-out method became established in Japan. Another technological advancement from Japan is recycling. However, this recycling does not mean that it is recovered from end users. It is recovered and refined from an intermediate process of a customer’s company that uses iodine (Figure 1).
Figure 1 Recycle the collection from a customer’s process. (From “Ise Chemicals Corporation Home Page”)
For example, recovery from processes of X-ray contrast media, a polarizing film, a fluorine compound, is typical. Such recycling began gradually around 1960. The volume of recycling is increasing.
Furthermore, spherical iodine (1983, patent published) is also technology from Japan. In general, the shape of iodine is similar to a flake. The spherical iodine(prill) made it easier to handle iodine. This development demonstrates the high technological level of iodine in Japan (Photo 4).
Photo 4 : Iodine, flake and spherical
Flake-shaped iodine on the left. Spherical iodine(prill) on the right
Owing to the spherical shape, the particles do not easily stick to each other, and easily come apart if a certain force is applied.
[Provided by Ise Chemicals Corporation]
4-6 Chile is No.1 again
In the 1990s, price competition with Chilean producers was intense. Japanese iodine makers faced a difficult period. Chile’s saltpeter and iodine resources are a few meters below the surface of the desert. They are extracted using a kind of open-pit mining. Of course, subsequent processes for final products are necessary; however, the period and amount of capital investment are relatively shorter and smaller than brine iodine. Therefore, the increase in production was easy to manage. Consequently, price competition often happened historically.
Chile returned to the No.1 producer of iodine in the world in 1998(Graph 5).
Graph 5 : From “U.S. Geological Survey” data
5.Transition in use of iodine
5-1 Expansion to various fields
Despite some fluctuations, in the long run, the global demand for iodine is constantly growing. Before World War II, the demand for iodine was centered on its use as medicine and disinfectants. However, in recent decades, the demand is varied, such as X-ray contrast media, polarizing films, catalysts, pharmaceuticals, salt additives, feed additives, and heat resistant stabilizers. This has led to an average annual growth rate of 3% in the world over the past a few decades. As about 40% of iodine is exported and used overseas (80% exported in the past), it is necessary to consider the transition in its use not only in Japan but also globally.
5-2 Disinfectants/antiseptics
Iodine was discovered in 1811. Its scholarly paper was published in 1813 and it was named as an element the following year. Its medical properties were known by 1820. Coindet, a doctor in Geneva, Switzerland, made an alcohol solution of potassium iodide and iodine, which is a tincture of iodine. He then used it to treat goiter caused by lack of iodine in the body. However, when used as internal medicine, iodine had a strong irritant effect on the stomach and did not become popular as a treatment method.
However, the tincture of iodine itself became popular in the form of disinfectants/antiseptics. Around 1830, Lugol, a French doctor, seemed to use it not only as a curative for goiter but also as a disinfectant for injury. Subsequently, this disinfectant/antiseptic application became mainstream. In Japan, until the Meiji/ the Taisho/middle of the Showa eras, the tincture of iodine was generally used as a disinfectant/antiseptic for injury or surgery (Photo 5).
Photo 5 : A glass container on the right.
A wooden cover on the middle and left.
“Tincture of Iodine” is written on the top of a wooden cover.
(Before World War II)
[Collection of The Society of Iodine Science]
In addition, iodine tincture was also used for animals. Photos 6,7,8, and 9 are ampoules of iodine tincture, which were used as drugs for war horses.
Photo 6 : A box containing 10 ampoules.
On the label, “Iodine Tincture Ampoules”
“Army Veterinary Materials Headquarters”
[ Collection of the Society of Iodine Science]
Photo 7 : Opening the box. Contains 10 ampoules with gauze.
Photo 8 : Instructions for use, on the back of the box lid.
The instruction is to crush the tip of an ampoule, moisten the
gauze with iodine tincture, and apply to the wound.
Photo 9 : An ampoule, labeled “IodineTincture2.0cc.”
After the war, in 1949, povidone-iodine, in which iodine is dissolved to polyvinylpyrrolidone (PVP), appeared as medical disinfectants/antiseptics (29). This greatly reduced the irritation caused by iodine tincture, and became the mainstay of iodine based-chemicals for this application. ” Isodine” is a well-known povidone-iodine, used in a gargle. Povidone-iodine is also widely used for disinfection during surgery.
Iodophor is a disinfectant consisting of iodine, surfactant, and PVP. It functions as a disinfectant/antiseptic in a wide range of applications such as the food industry, agriculture, fishery, and environment.
5-3 Almighty medicine
The function of disinfectants/antiseptics of iodine gradually spread globally as iodine tincture, and so on. From the mid-19th century to the mid-20th century, it was widely used as the almighty medicine for treating various diseases. For example, in Tolstoy’s Anna Karenina, a man performs iodine-inhalation to cure pulmonary tuberculosis (30).
Around the mid-Taisho period, the popularity of iodine as a specific remedy for various diseases increased. In 1921, in Japan, various commentaries on the efficacy of iodine were published in succession. These were not written by a public institution, but by private doctors. However, the authors did overestimate the efficacy of iodine.
For example, “The Explanation of Makino’s Iodine” (31), published in April 1921, details the use and effect of iodine on tuberculosis, pleurisy, asthma, pneumonia, flu, syphilis, gonorrhea, diabetes, and so on. In November of the same year, “Iodine Therapy” (32) was issued. It made similar statements, although its explanation of the efficacy of iodine was more limited than “The Explanation of Makino’s Iodine.”
These books also contained examples of iodine preparations, such as intravenous injection solution, oral liquid, anti-flatulent, anti-inflammatory-ointment, burn-ointment, and so on. It seemed that the main component of the injection solution and oral liquid was generally potassium iodide.
Photo 10 is an example of iodine being sold as a drug for rheumatism, Photo 11 is an example of iodine being sold as a drug for syphilis, gonorrhea, and so on.
Photo 10 : A bag of iodine tablets, before World War II. The description states “Quick Acting medicine for acute and chronic rheumatism.”
(Sold at Honpo Fujiya Bunzo)
[Collection of The Society of Iodine Science]
Photo 11 : Postcard style advertisement.
At the top, the description states “Good for syphilis, gonorrhea, fever.”
(Made by Harasanbondo Pharmacy)
[Collection of The Society of Iodine Science]
Photo12,13 are a wooden box (for one dozen) and an advertisement of Blutose (trade name) sold by Fujisawa Tomokichi Shoten, as an analeptic. One of several types of Blutose is Jod Blutose. It is described as “Ideal iron-iodine preparation in which iodine is combined organically in solution of sodium ferroprotein. It contains organic iron and iodine 0.3% each. It is an analeptic with a special effect on lymphatic temperament.”
Photo 12 (Left) : Jod Blutose one dozen wooden box
[Collection of The Society of Iodine Science]
Photo13 (Right): An advertisement of Blutose
From “Academic Specimen Digital Archive
The University Museum, The University of Tokyo”
5-4 X-ray contrast media
According to the History of Radiological Technology in Japan(33), X-ray contrast media has a long history. It is said that in 1896, immediately after the discovery of X-rays in 1895, gypsum was injected into a corpse to image the blood vessels using X-rays, in Italy.
The basic principle of contrast media is to absorb X-rays and display images of blood vessels or organs in the body. However, the development of contrast media for living bodies is also the history of fighting its side effects. Contrast media for digestive system were tried around 1900. Although various materials such as bismuth were tried; however, there was always the danger of poisoning. In 1913, Krause prescribed barium sulfate. This is a stable compound with little risk of poisoning even when used in a large amounts, and it is inexpensive. Therefore, it is in use even today.
After the digestive system, urological contrast media were tried. In 1905, Lichtenberg and Volker performed imaging of the renal pelvis using solutions of collargolum and colloidal silver iodide. Thus, iodine appeared in this field. It helped obtain an image with good contrast. However, very careful attention was required, because these contrast media had high injection pressures of the solution, with the danger of poisoning due to defective chemicals.
In the early 1920s, Sicard succeeded in myelography by lipiodol (a mixture of iodine and poppy oil). Later, lipiodol was also used in the imaging of brain ventricle, uterus, fallopian tube, bronchi. In 1931, Daiichi Pharmaceutical Co., Ltd. marketed iodo-oil in Japan.
In parallel with oil-based contrast media, there was development of aqueous contrast media, which can be injected by intravenous injection. Swick performed the first excretory urography with sodium pyridine iodide.
In 1930, Sugii, at the University of Tokyo, announced a method of synthesizing pyridine-iodide-based contrast media. It was subsequently released by Daiichi Pharmaceutical Co., Ltd. as “Sugiuron.”
In the 1950s, after the war, a triiodo compound in which three iodine were linked to a benzene ring was developed. Before this, the iodine compound was composed of one or two iodine. Subsequently, this triiodo compound became the mainstream. The increased iodine content in a molecule improved imaging capability. However, the biggest drawback of contrast media in this generation was the high osmotic pressure due to ion dissociation. It was a major cause of side effects.
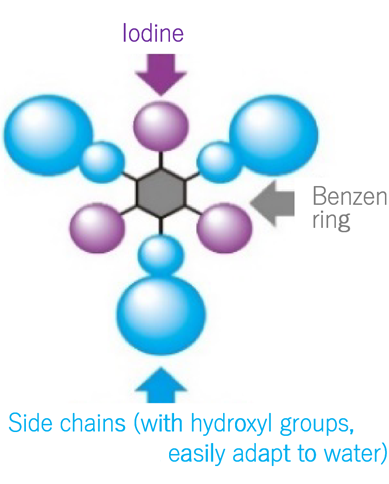
Today, nonionic contrast media have been developed. It has become possible to image at low osmotic pressure (Figure 2 and 3). Consequently, the side effects of contrast media have significantly reduced. Japanese research played an important role in proving their safety.
Figure 2: Three iodine were linked to a benzene ring, which improved imaging capability. Nonionic side chains (which had hydroxyl groups and easily adapted to water) were introduced. This reduced osmotic pressure.
During the transition period from ionic contrast media to non-ionic contrast media, doctors of roentgenology voluntarily created a committee to study the side effects of contrast media. The committee collected data on 170,000 cases of ionic contrast media and non-ionic contrast media respectively, and analyzed them. This study was highly evaluated internationally (34).
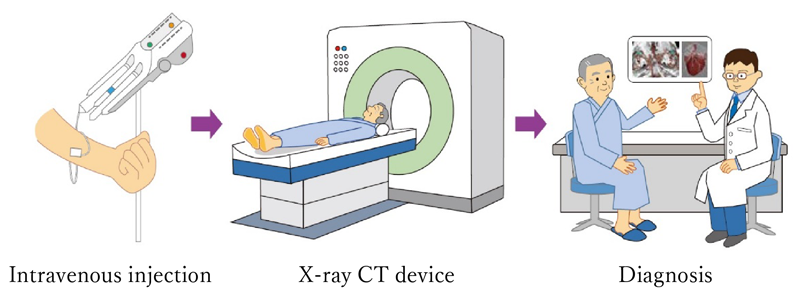
Figure 3 : How to use X-ray contrast media
Generally, contrast media injected by intravenous injection. Then an image is taken with an X-ray CT device followed by a diagnosis. It is used to examine blood vessels such as the heart, brain, kidneys, and liver.
Today, imaging is done by X-ray CT, MRI, ultrasound, and so on. It is performed by making the best use of each characteristic. Of these, only X-ray CT uses iodine for contrast media. However, the number of people using X-ray CT is steadily increasing every year. More than 20% of the global demand for iodine is for contrast media applications. Countries produce contrast media now are limited to the countries of Europe, the United States, and China. Japan does not produce contrast media.
5-5 Medicines for thyroid disorders and iodized salts for prevention of thyroid disorders
As mentioned, iodine as medicine was used to treat goiter; however, it did not become a popular medicine for treating thyroid disorders. However, in the 20th century, potassium iodide or potassium iodate became widely used in the treatment of goiter and cretinism (5).
Figure 4:Lack of iodine causes thyroid hypertrophy or goiter.
[Provided by Mr. Tatsuo Kaiho]
In addition, the use of iodized salt for prevention of goiter was first proposed by a French chemist named Boussingault, in 1831. From 1916 to 1921, US pathologists Marine and Kimball observed the use of iodized salt. Consequently, adding iodine to salt was decided at the national level (35). The first such iodized salt was released as a product in the United States in 1924. Now, table salt infused with potassium iodide, potassium iodate, or, sodium iodide is sold in the United States, Europe, landlocked countries, and regions where iodine components are leaching away from the surface (Figure 5).
Figure 5 : Table salt containing iodine.
Often sold in a 700 gram (25 ounce) can.
However, many regions still have iodine deficiency disorders. The International Council for Control of Iodine Deficiency Disorders (ICCIDD) was established in 1986. Eradication activities for iodine deficiency disorders have been promoted in cooperation with WHO, UNICEF. Although it has not reached a significant level of achievement yet, the Foundation for Growth Science is playing a central role in supporting these activities in Japan (36).
Since 1997, to support these movements, companies, local governments in Chiba, Niigata, which are the main iodine-producing regions, have donated and supplied iodine to countries where iodine deficiency disorders are common(Photo 14).
Photo 14 : Iodine (potassium iodate) Presentation Ceremony to
the Republic of Madagascar
October 29, 2018 at Chiba Prefectural Office
5-6 Photographic film material
Silver halide is used as photosensitive material in film.
It was used in France in the 1830s for the first time. As a product, it had stable demand before digital cameras became mainstream. A gel containing silver iodide crystals or silver bromide crystals was applied to the surface of film for it to function as photosensitive material. Silver iodide is said to be advantageous for high sensitivity. Although the demand of iodine for photographic film material is minor now, the technology of photosensitive material has a history of development involving iodine.
5-7 Halogen light
When an incandescent light bulb is lit at high temperature, high illuminance can be obtained and downsized. However, a simple increase in temperature shortens the life of a tungsten filament. A longer lifespan can be obtained by infusing inert gas and a small amount of halogen, such as iodine, into a light bulb.
Halogen lights have been used since around 1960 in automobile headlights, runway lighting, home downlights, and photographic lights (Photo15).
However, the emergence of LEDs, which save significant energy, has led to rivalry.
Photo 15 : An early halogen light as a camera strobe
[Collection of The Society of Iodine Science]
5-8 Catalyst
Iodine, which has good reactivity, also acts as s a catalyst.
Iodine has been used as a catalyst for a long time. However, a relatively new example of its large-scale use is of a coating agent that repels water and oil (water and oil repellant), which appeared around 1960, and is made of fluoropolymers. The fluoropolymers with such performance are made by a series of reactions (telomerization) using iodine. As iodine does not remain in the final product, it plays a catalytic function. In addition, from about 1970, a catalyst composed by iodine and iridium has been used for making acetic acid industrially. Consequently, acetic acid can be efficiently produced under the conditions of low pressure and low temperature.
5-9 Polarizing film
The polarizing phenomenon is that light with one direction of vibration can be passed and the other is absorbed. This phenomenon was discovered at the end of the 17th century; however, the technology of industrial production for polarizing film was developed in the United States from 1920 to 1940. Thus, high polarization was achieved by absorbing iodine on a thin film of PVA (polyvinyl alcohol) and stretching it. Almost simultaneously, polarizing film using dye instead of iodine was developed. Both types of polarizing film have been developing. However, in the field of TVs and smartphones, which require high polarization and high transparency, iodine type polarizing film is the mainstream.
Polarizing film began to be used in liquid crystal displays (LCDs) around 1960. It spread to watches or various measuring instruments. Then, it was also used in TVs and smartphones (Figure 6).
From 1980 to 2000, when LCDs developed, Japan was at the forefront of the electronics industry. Therefore, research by Japanese chemical and electronics companies contributed significantly to improving the performance of polarizing film.
Today, about 10% of the iodine demand is for polarizing film.
Figure 6 : Polarizing film is used in LCDs in TVs, smartphones, and PCs.
5-10 Stable iodine preparations
“Radioactive iodine (iodine 131[atomic weight 131])” diffuses in the event of a nuclear disaster. If taken into the body, it accumulates in the thyroid and causes internal radiation exposure. To prevent this, normal iodine (stable iodine, atomic weight 127), which does not contain radiation, is used immediately after a nuclear disaster. By administrating stable iodine preparations (Photo 16), the iodine in the thyroid becomes almost saturated, the accumulation of radioactive iodine in the thyroid is suppressed, and most of it is excreted.
Photo 16 : Stable iodine preparations(37)
Administration of stable iodine, as an emergency protective measure, was described in papers since the 1960s. Following the Chernobyl nuclear accident, WHO established guidelines, and Japan too established operation rules.
5-11 Medical use of radioactive iodine
The medical use of radioactive iodine also began. In Japan, such medicine began to be used around 1960. These medicines use radioactive iodine which is an isotope [* 1] of normal iodine (stable iodine, atomic weight 127). Specifically, they are iodine 123(atomic weight 123), iodine 125(atomic weight 125), iodine 131(atomic weight 131), and so on.
Iodine 123 is suitable for diagnosis because it has a short half-life [* 2](13.2 hours) among medical radioactive iodine, and it has reasonable radiation energy. Thus, it is used for obtaining an image through emitted radiation. Such images can be used to diagnose Lewy body dementia and Parkinson’s disease by imaging blood flow in the brain.
Iodine 125 has a long half-life (59.4 days), and it emits weak radiation energy continuously. For example, in the treatment of prostate cancer, a capsule containing iodine 125 is inserted into the focus. Cancer cells are burned by the emitted radiation.
Iodine 131 has a medium half-life (8 days), and it is used for diagnosis and treatment. Sodium iodide, containing iodine 131, is the earliest therapeutic radiopharmaceutical. It is orally administrated to treat hyperthyroidism or goiter (38).
[* 1] isotope: An element whose number of protons in the nucleus is the same, but whose mass number is different, due to the difference in the number of neutrons.
[* 2] half-life: The period until the radioactive element is reduced to half due to decay.
5-12 The Nobel Prize and iodine
In 2000, Dr. Hideki Shirakawa et al. won the Nobel Prize for the invention of conductive polymers.
They discovered that electricity passes through when a very small amount of iodine is added to polyacetylene. This principle led to the development of conductive polymers used in smartphone touch panels, among other uses. Its impact on our lives has been significant.
Iodine is also active in a cross-coupling reaction, which connects two different types of molecules together. In 2010, Dr. Akira Suzuki and Dr. Eiichi Negishi et al. were awarded the Nobel Prize for the development of this cross-coupling reaction. The catalyst as the protagonist this time was palladium; however, iodine was used in the previous step of the reaction.
6.Closing remarks
In terms of future demand, the function of iodine to ”connect molecules(cross-coupling),” “make polymers (polymerization control agent),” ”function as solar cell material(perovskite-type crystal),” and so on, are expected.
In history, iodine has expanded its range of activities from disinfectants/antiseptics, to various purposes today. While some applications are on the verge of completing their lifecycle, new applications are emerging because of iodine’s responsiveness. Iodine will be useful in various situations in the future.
7.Chronology
(Italicized blue letters indicate events overseas)
| 1811 | Discovery of iodine in France |
| Around 1820 | Coindet, a medical doctor in Switzerland uses iodine tincture (an alcoholic solution of iodine) to treat thyroid. |
| 1830 around | Lugol, a medical doctor in France, began using iodine tincture as disinfectant for wounds. |
| 1830s | Silver iodide began to be used as photosensitive material for film in France. |
| 1842 | Import of iodine begins in Nagasaki. |
| 1846 | The first successful extraction of iodine by a Japanese. |
| Around 1857 | Yukichi Fukuzawa also attempts to extract iodine in Tekijuku, Osaka. |
| 1861 | Manufacture of iodine compounds at the shogunate’s pharmaceutical factory. |
| 1862 | Discovery that oil field brine contains a large amount of iodine, in Java, Indonesia. |
| 1868 | Chile began exporting iodine as a by-product of saltpeter. |
| Around 1873-1874 | Trial of iodine production begins in Hokkaido. |
| 1877 | Enactment of Poisonous and Deleterious Substances Control Act (including iodine) by the Meiji government. |
| 1884 | Trial of iodine production begins in Mie prefecture. |
| 1887 | Chujiro Kase establishes an iodine factory in Tokyo(start of full-scale iodine business in Japan). |
| 1888 | Suzuki family( founding family of Ajinomoto Co., Inc.) begins producing iodine in Kanagawa prefecture. |
| 1890 | Chobei Takeda, Gohei Tanabe, and Gisaburo Shiono establish Kogyosha in Doshomachi Osaka and start iodine production. |
| 1893 | Toragoro Tanahashi acquires a patent for potassium iodide manufacturing in Tokyo. He establishes Azabu Yosho Joint Stock Company, which starts iodine production. |
| 1901 | Nobuteru Mori(founder of Showa Denko K.K.) starts apprenticeship at Ikehira Crude Iodine Factory |
| 1904-1905 | The Russo-Japanese War. Increase in demand for iodine as disinfectants/antiseptics and demand for potassium chloride(co-products) as raw material for explosives. The iodine business grows significantly. |
| 1905 | X-ray contrast media using iodine appears in Europe. |
| 1907 | Chujiro Kase, Toragoro Tanahashi, Saburosuke Suzuki jointly establish Nippon Chemical Industrial Co.,Ltd. |
| 1908 | Nobuteru Mori establishes Sobo Marine Products K.K. |
| 1912 | Enkichi Ishihara, as a leader, establishes Mie Iodine Manufacturing Co., Ltd. |
| 1919 | Iodine production in Japan recorded at 253tons. (maximum until World War II) |
| 1924 | Sales of iodized salts for prevention of thyroid disorders begins in the United Sates. |
| 1926 | Nobuetru Mori establishes Nihon Iodine K.K. (today, Showa Denko K.K.) |
| 1927 | Ise Iodine Factory (today, Ise Chemicals Corporation) established. |
| 1928 | Construction of plant to produce iodine from brine in the United States. |
| 1934 | Mikasa Shokai (today, Godo Shigen Co.,Ltd.) constructs an iodine factory in Isumi county, Chiba prefecture (beginning of iodine business using natural gas brine in Japan). |
| 1938 | Tennen Gas Kogyo (today, Kanto Natural Gas Development Co., Ltd.) constructs an iodine factory in Mobara, Chiba prefecture. |
| 1939 | Nippoh Kagaku Kogyo (today, Nippoh Chemicals Co.,Ltd.) constructs an iodine factory in Isumi county, Chiba prefecture. |
| 1940 | Nihon Kansui Kogyo Kenkyusho (today, Nihon Tennen Gas Co., Ltd.) begins iodine production at Seki Mura in Chiba prefecture. |
| Around 1920-1940 | Development of polarizing film using iodine progressing in the United States. |
| 1949 | Ise Chemicals Corporation decides to move its production base from Mie prefecture to Chiba prefecture. |
| Around 1950 | Production of seaweed iodine ends. |
| 1952 | Aiming at mass production of brine iodine, some iodine production companies begin trial of the blow-out method. |
| Around 1960 | Industrial mass use of iodine as a catalyst and iodine recycling begin. Beginning of research on the administration of stable iodine preparations (prevention of internal radiation exposure in the event of nuclear disaster) Commencement of use of pharmaceutical products that utilize radioactive iodine. |
| 1961 | Ise Chemicals Corporation adopts the blow-out method full-scale. |
| 1966 | Nihon Halogen Co.,Ltd.,(today, JX Nippon Oil & Gas Exploration Corporation) begins iodine production in Niigata prefecture. |
| 1967 | Japan becomes the No.1 iodine producing country in the world, surpassing Chile. |
| 1970 | Teikoku Oil Co., Ltd(today, INPEX Corporation) enters the iodine business. |
| 1975 | Ise Chemicals Corporation begins iodine production in Miyazaki prefecture. |
| 1983 | Development of spherical iodine. |
| 1995 | Toho Earthtech,Inc. begins iodine production in Niigata prefecture. |
| 1998 | Chile surpasses Japan to become the largest producer of iodine globally, again. |
| 2000 | Dr. Hideki Shirakawa et al. receive the Nobel Prize for the invention of conductive polymers.(the addition of iodine is the deciding factor in making the polymers conductive) |
| 2010 | Dr. Akira Suzuki and Dr. Eiichi Negishi et.al. receive the Nobel Prize for the development of cross-coupling reaction (iodine is used in the previous step of the reaction). |
8.Postscript
In preparing this article, I received much cooperation from many people. For example, companies of the Japan Iodine Industry Association sent us company histories and several materials. Dr. Hirofumi Kanoh, Chair of The Society of Iodine Science(SIS), Dr. Tatsuo Kaiho, Director of SIS, Mr. Akira Sakuma, Special Member of SIS, Dr. Yozen Fuse, The Foundation for Growth Science, gave me valuable advice at the draft stage. In addition, regarding the collection and survey of historical documents and property, Mr. Hidenori Suzuki and Mr. Satoshi Asakura, both members of SIS, made great efforts. Mr. Kazuhiko Saito, Secretary General of SIS, supported me in various administrative procedures. I would like to thank everyone again.
March 2020
Takashi Fujino
Special Member of The Society of Iodine Science
9.Bibliography
- 1) Gaiten Yoneda editing “Osaka to Kusuri” Osaka University Press 2002 P.28
- 2) Youan Udagawa Seimi-Kaiso external-book volume 1 1847
[The relevant description is in the excursus of external-book volume 1] - ”Kakufuken Juyo Shohin Chosa Hokoku”
Commerce Bureau of Ministry of Agriculture and Commerce 1912 P.113 - Yukichi Fukuzawa ”Fukuo Jiden” 1899 [Concerned matter is written in the latter part of the experiment section for making hydrochloric acid]
- Tatsuo Kaiho “Tokoton Yasashii Yoso no Hon” 2015 P.14, 28, 113
- Sakae Ito “Ito Genboku Den” 1916 P.57
- Tatsuo Hirai editing “Yakugyo 50 nenshi” 1927 P.23-25
- ”Meiji Kogyoshi Kagakuhen” Nihon Kogakukai 1925 P.1012-1013
- Rokuro Takahashi “Hokkaido ni Okeru Yodo oyobi Enkakari Seizou ni Kansuru Chosa” Otaru Kouto Shougyo Gakko 1918 P.3
- Kiyoshi Sakamoto “Mieken Yodo Seizougyo no Rekishi” Suisan Kenkyushi volume 7 Suisan Kouyu Kai 1912 P.12
- Yozen Fuse et al. “The History of Industrial Production of Iodine in Japan”
Journal of The Society of Iodine Science No.21 2018 P.96 - Keiju Ishihara “Mieken Shima Chihou ni okeru Meijiki no Yodo Sangyo” Umi to Ningen (Journal of Toba Sea-Folk Museum) (No.29) 2006 P.58
- Ginjiro Aikawa, Kintaro Okamura “Suisan Kogei Yodo Seizo Shinsho” 1892
- Toyokichi Takamatsu, Keizo Tanba, Yoshizumi Tahara editing “Kagaku kogyo Zensho book 1” 1904 P6-7
- ” Aji wo Tagayasu – Ajinomoto 80 nenshi-” 1990 P.45
- ” Showa Denko 50 nenshi” 1977 P.7
- ” Zukai Kagaku Kogyo” Kagaku Chishiki Fukyukai 1929 P.51
- ” Mieken Yakugyoshi” 1940 P.220
- ” Kojo Toukeihyo”(Statistical survey by the Ministry of Agriculture and Commerce, and, the Ministry of Commerce and Industry)
- Armin Lauterbach, SQM S.A., Chile “Production Technology of Iodine in Chile” Forum on Iodine Utilization Journal No.2 1999 P.29
- Tetsuya Ishikawa “Wagakuni ni okeru Shinko Yodo Kogyo”
The Chemical Society of Japan Journal 63(2) 1942 P.165 - Shigeru Sunagawa “The Journal of the Japanese Society for the History of Chemistry Volume 24” 1997 P.25
- “Chika Shigen to Tomoni Ikiru “
75-year company history of Godo Shigen Co., Ltd. 2009 P.205, 212 - ”50 nen no Ayumi”
50-year companies histories of Kanto Natural Gas Development Co., Ltd. and Otaki Natural Gas Co., Ltd. 1981 P.340, 356 - ”Nippoh Kagaku 50 nen no Ayumi”
Nippoh Chemicals Co.,Ltd. 1998 P.21, P.65-67 - ”50 nen no Ayumi” Nihon Tennen Gas Kogyo 1990 P34,55
- Shoichi Izawa, Takahiko Yamaguchi “Shuso Kogyo no Rekishi to Kongo no
Doukou” Chemical Engineering of Japan Volume 36 No.6 1972 P.40-41 - ”Sogyo 50 Nenshi” Toho Earthtech, Inc. 2007 P.155
- Yoshiki Hatazaki “The Journal of the Japanese Society for the History of
Chemistry Volume 27 No.4” 2000 P.6 - Lev Tolstoy “Anna Karenina” chapter 20/book 5 1877
- Tamizo Makino “Makino Yodo no Setsumei” Shiritsu Dainippon Makino Yodo Kenkyusho Kansai Shibu 1921
- Kumao Hayashi “Yodo Ryoho ni Tsuite” Teikoku Yodo Kenkyusho 1921
- ” Nihon Hoshasen Gijutsushi” editing by the Japanese Society of Radiological Technology 1989 P.208-217 P349-355
- Koichi Yamaguchi “Progress of X-ray Contrast Media and Adverse Reaction”
Forum on Iodine Utilization Journal No.2 1999 P.21-24 - Yozen Fuse “Yoso no Seirisayo to Kojosenshikkan Nihonjin no Yoso Sesshuryo” Shoku to Iryo Volume 7 2018 P.76
- Minoru Irie “Iodine Deficiency Disorders”
Forum on Iodine Utilization Journal No.3 2000 P.13-16 - ” SIS Letters No.15” 2014 The Society of Iodine Science
( updated with the latest picture) - Norifumi Shirakami “Medical utility of iodine and radioactive iodine”
The Society of Iodine Science Journal 14 2011 P.14-17
In addition, figure 2,3,5, and 6 are reprinted from “Nihon ni Takusan Aru Shigen te Nandaro? Sore ha Yoso” (published by SIS in February 2020)
10.Comparison list of unique nouns (English-Japanese)
■2-1
Ryuho Shima:島立甫
Seimi-Kaiso:舎密開宗
Yukichi Fukuzawa:福沢諭吉
Koan Ogata:緒方洪庵
Fukuzawa’s autobiography:福翁自伝
Genboku Ito:伊東玄朴
Iemochi Tokugawa:徳川家茂
Kazunomiya:皇女和宮
■2-2
Seisuke Rokkado:六花堂清助
Manpei Kashima:鹿島萬平
Seikichi Okamoto:岡本政吉
Sakubei Yamamoto:山本作兵衛
Taizo Tamagawa:玉川泰三
Chujiro Kase:加瀬忠次郎
Tomomi Iwakura:岩倉具視
Chobei Takeda:武田長兵衛
Gohei Tanabe:田辺五兵衛
Gisaburo Shiono:塩野義三郎
Toragoro Tanahashi:棚橋寅五郎
Shunrei Murata:村田春齢
Kahei Tomoda:友田嘉兵衛
Saburosuke Suzuki:鈴木三郎助
Nobuteru Mori:森矗昶
■3-2
Enkichi Ishihara:石原圓吉
■5-2
Isodine:イソジン
■5-3
“The Explanation of Makino’s Iodine”:「牧野沃度の説明」
“Iodine Therapy”:「沃度療法について」
Honpo Fujiya Bunzo:本舗冨士谷文蔵
Harasanbondo Pharmacy:原三盆堂薬局
Blutose:ブルトーゼ
Fujisawa Tomokichi Shoten:藤澤友吉商店
■5-4
Sugii:杉井義雄
5-12
Dr. Hideki Shirakawa:白川英樹博士
Dr. Akira Suzuki:鈴木章博士
Dr. Eiichi Negishi:根岸英一博士
- 1.Introduction
- 2-1 The dawn of iodine in Japan - Edo period
- 2-2 The dawn of iodine in Japan - After the Meiji Restoration to the beginning of the 20th century.
- 3-1 Development of the iodine industry - Boom due to wars
- 3-2 Development of the iodine industry - Industry reorganization after the Russo-Japanese War
- 3-3 Development of the iodine industry - Evolution of the monopoly system for by-products
- 3-4 Development of the iodine industry - Impact of World War I
- 3-5 Development of the iodine industry - Industry hardship and increasing production for World War II
- 4-1 Change in material - History of non-seaweed iodine production
- 4-2 Change in material - Development in Chiba
- 4-3 Change in material - Development of natural gas industry and innovation in production methods
- 4-4 Change in material - Increasing production to the top of the world
- 4-5 Change in material - Technology development in Japan
- 4-6 Change in material - Chile is No.1 again
- 5-1 Transition in use of iodine - Expansion to various fields
- 5-2 Transition in use of iodine - Disinfectants/antiseptics
- 5-3 Transition in use of iodine - Almighty medicine
- 5-4 Transition in use of iodine - X-ray contrast media
- 5-5 Transition in use of iodine - Medicines for thyroid disorders and iodized salts for prevention of thyroid disorders
- 5-6 Transition in use of iodine - Photographic film material
- 5-7 Transition in use of iodine - Halogen light
- 5-8 Transition in use of iodine - Catalyst
- 5-9 Transition in use of iodine - Polarizing film
- 5-10 Transition in use of iodine - Stable iodine preparations
- 5-11 Transition in use of iodine - Medical use of radioactive iodine
- 5-12 Transition in use of iodine - The Nobel Prize and iodine
- 6.Closing remarks
- 7.Chronology
- 8.Postscript
- 9.Bibliography
- 10.Comparison list of unique nouns (English-Japanese)
- 11.Map of Japan

![海藻を採取・乾燥させる様子[図解化学工業 (17)]より](/wp/wp-content/themes/sis/history/images/history_pic03.png)


![写真5:戦前のヨードチンキのガラス容器[右]とその外筒(木製)](/wp/wp-content/themes/sis/history/images/history_pic11.png)
![写真6:アンプル10本入りの箱。[ヨウ素学会 蔵]](/wp/wp-content/themes/sis/history/images/history_pic12.png)



![写真13(右側):ブルトーゼ広告 [東京大学総合研究博物館学術標本デジタル・アーカイブより]](/wp/wp-content/themes/sis/history/images/history_pic25.png)
![図4:[海宝龍夫氏 提供]](/wp/wp-content/themes/sis/history/images/en/history_pic18.png)